

Oriented cell division is critical for stem cell maintenance, generating cellular diversity, morphogenesis and preserving tissue organization. The axis of cell division is determined by orientation of the mitotic spindle, which is achieved via a wide range of mechanisms, many, but not all, of which converge upon localized cortical pulling forces on astral microtubules. In polarized cells, asymmetric cytokinesis, wherein contractile ring closure is biased towards the apical surface, also appears to be a conserved feature of cell division. While mechanisms of spindle orientation and cytokinesis have been studied extensively in cell culture models, how these processes are regulated by tissue architecture in vivo is an exciting new frontier.
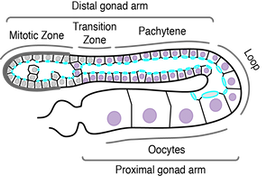
C. elegans GSCs provide a novel system in which to ask how tissue organization regulates cell division. The C. elegans germline is a syncitial, tube-like organ, with GSCs arranged in a polarized, circumferential monolayer, with actin binding proteins and contractility regulators enriched on the lumenal face. GSC polarization is distinct, in that a stable intercellular bridge occupies the lumenal surface linking GSCs to an internal shared cytoplasmic core (the rachis). Additionally, unlike most epithelia, GSCs do not appear to be connected by lateral cell-cell junctions.


Using high-resolution live imaging and quantitative image analysis, we have shown that GSCs exhibit planar spindle orientation, such that cells tend to divide parallel to the surface of the gonad (modeled as a cylinder). GSCs also undergo asymmetric cytokinesis, with the cytokinetic ring closing off-center and toward the rachis surface. These results demonstrate the diversity of mitotic events that can be assayed in GSCs and lay the framework for future investigations into the mechanisms underlying both. In ongoing collaboration with the lab of Jean-Claude Labbé (Université of Montréal/IRIC), we have recently developed imaging conditions and an image/data-processing pipeline that allows for high-throughput analysis of GSC spindle dynamics relative to the rachis surface (modeled as a polygon mesh). We are now using this system to dissect the genetic regulation of GSC spindle orientation.
Future projects will investigate GSC spindle orientation along the rachis-basal axis of the cell, focusing on the role of cell geometry and mechanical forces. We are also interested in understanding intercellular bridge dynamics during GSC division, in particular, how the single mother bridge is converted into two, such that each daughter remains connected to the rachis. The C. elegans embryo has proved to be a seminal model for dissecting mechanisms underlying spindle orientation during asymmetric cell division and the C. elegans germline stands to make equal contributions to the emerging picture of how cell division shapes and is shaped by tissue architecture.